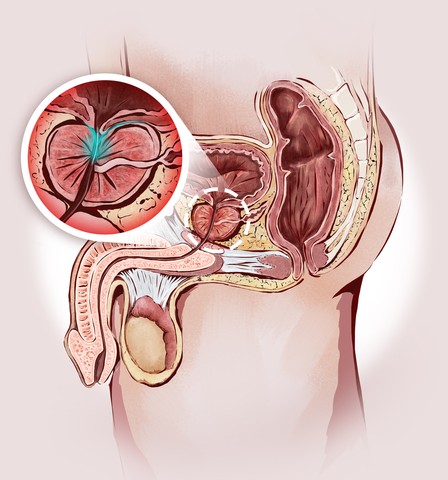
The role of androgens and androgen receptor signals in the inflammation-induced development of benign prostatic hyperplasia and prostate cancer, two conditions with different epidemiological and pathological features, is the focus of a new review study, “Androgen receptor and immune inflammation in benign prostatic hyperplasia and prostate cancer,” published in the journal Clinical Investigation.
Benign prostatic hyperplasia (BPH) and prostate cancer (PCa) are frequent diseases of middle-age and older men, and are associated with abnormal growth of the prostate.
The authors note that studies dating back to 1995 have associated androgen concentration and/or androgen receptor (AR) activity to BPH. More recently, androgen/androgen receptor (AR) signaling has been shown to contribute to the enlargement of lesions in both BPH and PCa, and the therapeutic blocking of this signaling pathway improves symptoms or reduces the lesions’ size. Data based on a BPH mouse model have suggested that stromal AR may function through epithelial-stromal interacting signals to promote BPH progression, as BPH consists of 88% stromal cells and 9% epithelial cells. Moreover, several studies have associated androgens/AR signals to inflammation and impact on BPH progression. Thus, targeting both AR and immune inflammation may become a reasonable therapeutic approach for BPH treatment.
Several clinical trials have also suggested that targeting both androgen/AR and α-1 adrenergic receptor signals contribute to the suppression of BPH development. However, androgen-deprivation therapies with surgical or medical castration or with anti-androgens are no longer a standard BPH treatment, as several trials testing luteinizing hormone-releasing hormone analog and anti-androgen flutamide led to adverse events. A better understanding of the disease etiology should allow for the development of more efficient treatments with reduced side effects.
For advanced metastatic prostate cancer treatment, the review found that androgen-deprivation therapy (ADT) composed of medical or surgical castration with or without anti-androgen is a first-line therapy. However, most patients treated with ADT alone develop castration-resistant PCa, i.e., they develop hormone ablation resistance a few years after starting ADT, with various side effects. At this stage of the disease, therapy alternatives are very limited. Until recently, the chemotherapeutic agent docetaxel represented the conventional therapy after castration resistance emerged, with a mean life extension in these patients of 2.9 months.
Overall, the authors state that androgen deprivation therapy alone may not be sufficient treatment for these two prostatic diseases due to its undesirable side effects. They conclude, “androgen/AR signals, especially those involved in inflammation, may play key roles to enhance cell growth in both stromal and epithelial cells for the promotion of BPH development. Targeting both AR and immune inflammation may become a reasonable therapeutic approach for treatment of BPH.”