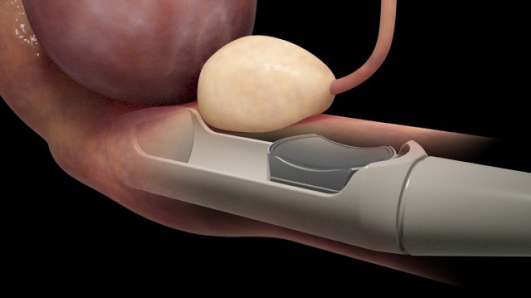
The U.S. Food and Drug Administration (FDA) has approved SonaCare Medical’s Sonablate 450 focused ultrasound system for ablation therapy on prostate tissue. A pioneer in high intensity focused ultrasound (HIFU) technologies, the Indianapolis, Indiana-based, SonaCare reports that it has received de novo clearance from the FDA to market the Sonablate 450 in the U.S., making it the first High Intensity Therapeutic Ultrasound (HITU) device to receive the agency’s regulatory authorization for prostate tissue ablation — a non-invasive, radiation-free technique used to treat localized prostate cancer. SonaCare Medical expects to begin U.S. distribution this month.
“The FDA regulatory authorization to market Sonablate in the U.S. is a milestone for non-invasive prostate care and a tremendous gain for men’s health,” says Mark Carol, MD, Chief Executive Officer of SonaCare Medical, in a release. “Men all over the world, in the more than 49 countries where it has already been authorized for use, have had access to this technology for prostate ablation. There are numerous peer reviewed articles attesting to its value in ablating the prostate while minimizing the occurrence of side effects. Our company is appreciative of the collaborative efforts made on the part of the FDA to bring this technology to the U.S.”
SonaCare Medical’s next-generation system for HIFU surgical prostate tissue ablation is powered by proprietary A3 Technology, which allows physicians to:
• Aim energy at specific tissue using integrated ultrasound imaging and sophisticated planning tools;
• Ablate targeted tissue with HIFU ablation that provides pinpoint accuracy while sparing untargeted tissue; and
• Analyze procedure results using real-time ultrasound imaging along with advanced tissue change monitoring software.
Focused ultrasound treatments are performed with no incisions, leading to few complications and minimal discomfort, enabling patients to return to daily activities rapidly.
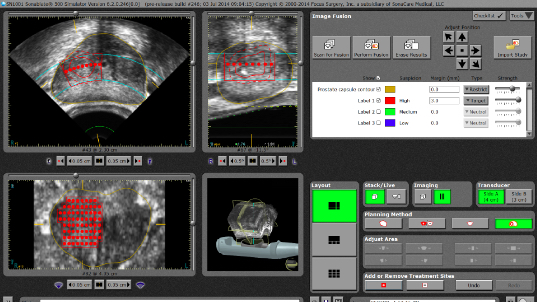
During HIFU therapy, ultrasound energy — sound waves — are focused at a specific location within the prostate called the focal point, where tissue temperature rapidly rises to almost 195 degrees Fahrenheit (90 degrees Celsius). Tissue at the focal point is destroyed, while the tissue located outside of the focal lesion remains unharmed. Because there is no radiation involved to destroy targeted tissue, the procedure can be repeated, if necessary. SonaCare’s proprietary Sonatherm HIFU Ablation Software utilizing T3 Technology enables the operator to target ablation zones with sophisticated planning and localization tools, treat with pinpoint accuracy while sparing untargeted tissue and track with advanced real-time ultrasound imaging.
A radio frequency (RF) signal is sent to a targeted ablation site prior to delivery of HIFU and then another signal is sent after delivery of HIFU to the same site. Tissue Change Monitoring (TCM) software calculates the change that took place and displays it on a monitor screen, quantifying tissue changes based on a comparison of RF ultrasound pulse-echo signals at each ablation site.
SonaCare says Sonablate is the only currently available device that enables physicians to routinely perform HIFU prostate ablations on prostates up to 40cc without previously performing a TURP (transurethral resection of the prostate) procedure. Sonablate features a fully integrated probe with dual ablation transducers that move robotically to follow the physician’s precise ablation plan. This image guidance allows for a customizable ablation plan tailored to each patient’s prostate diagnosis, and that allows the user to ablate in a wide variety of applications, including whole-gland and partial prostate ablation on men that have had or have not had previous prostate procedures.
Sonablate Clinical Trial
SonaCare Medical is currently conducting a clinical trial in the United States and Canada to determine the safety and efficacy of prostate cancer treatment using the Sonablate 450 with patients who have recurrent prostate cancer who have previously received treatment with external beam radiation therapy.
To be eligible for participation in the U.S. recurrent trials, candidates must have been treated with external beam radiation therapy two or more years prior; have low-risk, organ-confined recurrent prostate cancer T1c or T2a; be between the ages of 40 and 85; have a PSA level between 0.5ng/mL and 10ng/mL; and have a recent (within six months) prostate biopsy that is positive for cancer.
There is no cost to enroll in the study, nor does it require health insurance. For more information call 877.605.HIFU.
“I believe that we are at a pivotal point in prostate care,” comments Sonablate trial investigator http://urology.iupui.edu/about/adult/koch.php Michael Koch, MD, Chairman of the Department of Urology at Indiana University. “Simultaneous advances in imaging, fusion technologies, and now more focused therapies are going to allow us to precisely diagnose prostate conditions, and ablate these targeted areas rather than perform whole gland prostate surgery, which carries a significant burden on quality of life. HIFU will become the workhorse of subtotal prostate therapy.”
Herbert Lepor, MD , Professor and Martin Spatz Chairman in the Department of Urology at NYU School of Medicine, who also participated in the Sonablate trial, further explains: “HIFU is a novel technology that has been used worldwide to ablate prostate tissue. Until now, this technology was not available in the U.S. It is anticipated that ablative urological surgeons in the U.S. will quickly master and adapt this technology for their patients. HIFU offers the opportunity for the precise delivery of ablative energy to the prostate. Thus, it can be adapted to whole gland or focal gland ablation.”
“The FDA’s decision on Sonablate is an important step in providing men with prostate conditions access to this less invasive approach. We hope that focused ultrasound will eventually become a standard of care for treating the prostate,” says Neal Kassell, MD, a Distinguished Professor of Neurosurgery at the University of Virginia and Chairman of the Focused Ultrasound Foundation. “For men with conditions like prostate cancer, the option of a non-invasive procedure that can selectively target and treat diseased tissue is very appealing. American men have been traveling overseas for focused ultrasound treatment for prostate diseases for years, and we are pleased that they will now have access to this innovative treatment at leading centers in the United States.”
Sonablate is SonaCare’s second medical device to receive FDA regulatory authorization, complementing the 510(k) cleared Sonatherm laparoscopic HITU ablation device. “With the authorization to sell Sonablate in the U.S., we can now offer a full HIFU surgical suite of technologies working off of the same basic platform and providing benefits throughout health care centers,” says Mark Carol. “In addition, this de novo clearance, together with the CE Mark for Sonablate 500, will facilitate additional regulatory authorizations in markets beyond Europe and North America, further strengthening our world-wide market leadership in therapeutic HIFU devices.”
According to the Focused Ultrasound Foundation — a Charlottesville, Virginia-based, tax-exempt, high-performance, entrepreneurial service organization with a global reach that was created to improve the lives of millions of people with serious medical disorders by accelerating the development and adoption of focused ultrasound — more than 50,000 men around the world have been treated with focused ultrasound for prostate cancer. It is the leading clinical application of the technology, with more than 50 percent of patients who have undergone focused ultrasound around the globe having had prostate disease treated. The company notes that HIFU ablation technology has been successfully used to treat a wide variety of diagnoses, including benign prostatic hyperplasia (BPH), partial gland cancer, localized whole-gland prostate cancer, and recurrent prostate cancer.
In addition to Sonablate, EDAP’s Ablatherm focused ultrasound system for treating the prostate is under FDA review and may be approved soon. Focused ultrasound devices have been cleared to treat the prostate in more than 40 countries since the first approval in 2000. Several systems for focused ultrasound ablation of prostate tissue are commercially available or being researched in other geographic regions with differing guidance methods (ultrasound vs. magnetic resonance imaging) and approach (transrectal or transurethral).
Focused ultrasound is also currently approved in the U.S. to treat uterine fibroids and relieve pain from bone metastases, and there are a growing number of clinical applications in various stages of research and development around the world, including Parkinson’s disease, essential tremor, breast cancer, pancreatic cancer and brain tumors.
Working to clear the path to global adoption, the Focused Ultrasound Foundation coordinates and funds research, fosters collaboration, and builds awareness. The Foundation is dedicated to ensuring that focused ultrasound finds its place as a mainstream therapy for a range of conditions within years, not decades. Since its establishment in 2006, the Foundation has become the largest non-governmental source of funding for focused ultrasound research.
For more information, visit:
http://www.fusfoundation.org/
Sources:
Focused Ultrasound Foundation
SonaCare Medical LLC
US Food and Drug Administration (FDA)
EDAP TMS