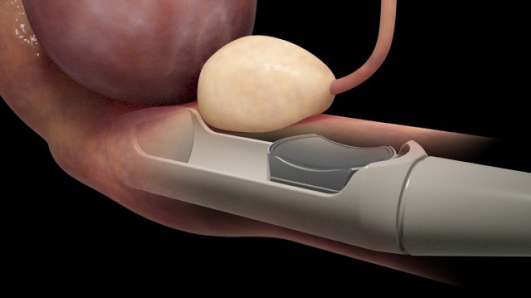
SonaCare Medical, LLC, announced that its minimally invasive and focused ultrasound technology for prostate tissue ablation has been successfully used in a first procedure at Texas Health Presbyterian Hospital in Dallas by an early advocate of the device, Dr. James Cochran.
Called Sonablate, the technology is the first high intensity therapeutic ultrasound (HITU) device to receive U.S. Food and Drug Administration (FDA) approval, according to a press release. The Sonablate HIFU is a non-invasive, radiation-free technique meant to treat localized prostate cancer. The next-generation system allows physicians to direct energy at targeted tissue using integrated ultrasound imaging and planning tools, and to analyze the results with real-time ultrasound imaging and tissue change monitoring software.
“My team and I are excited to be the first to offer Sonablate HIFU in Texas and the surrounding states,” Dr. Cochran said of the March 16 procedure. “We are exceedingly proud to be able to provide this minimally invasive prostate procedure to men who have been seeking an alternative option in prostate care. I am eager to witness the acceptance of HIFU ablation technology throughout the United States, and look forward to the additional advancements it inspires across an evolving medical landscape.”
Approved by the FDA in October 2015, Sonablate was introduced more than 15 years ago and has been used to treat nearly 15,000 patients in over 30 countries since then, including some 4,000 Americans who underwent the procedure outside the U.S. Dr. Cochran began using the HIFU device in 2005, and served as chief investigator for a SonaCare Medical clinical trial of HIFU as a treatment for benign prostatic hyperplasia (BPH) in 1999.
“Dr. Cochran has been one of the most committed long term supporters of focused ultrasound ablation in the U.S., treating patients at multiple locations outside the U.S. over the past 10+ years. I am personally delighted that James now has the opportunity to bring his experience with Sonablate to Dallas, Texas, where I am sure he will continue to further the development of prostate ablation using Sonablate as a robust clinical tool,” said SonaCare Medical’s chief executive officer, Dr. Mark Carol.
Sonablate was first used in the U.S. in a procedure at First Urology Kentucky in November 2015.