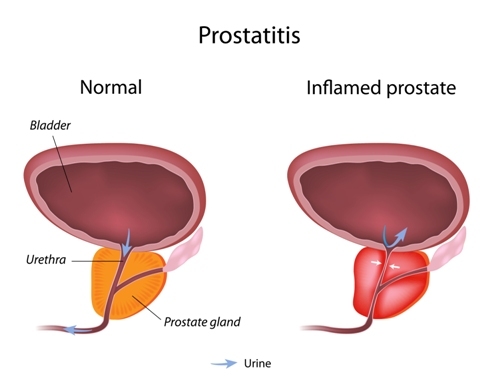
Novel therapeutic targeting of TLR4 signaling pathway may halt chronic inflammation leading to benign prostate hyperplasia (BPH), according to a study, “LPS/TLR4 Signaling Enhances TGF-β Response Through Downregulating BAMBI During Prostatic Hyperplasia,” published in the journal Scientific Reports.
Recent studies suggest that BPH development is linked to the accumulation of mesenchymal-like cells via a process of epithelial-mesenchymal transition (EMT), and in this case from prostatic epithelium. The EMT is key for an appropriated embryonic development, where epithelial cells switch back and forth between the epithelia and mesenchyme (mesenchymal cells are able to develop into different types of cells, including cells of the lymphatic and circulatory systems, as well as connective tissues throughout the body, such as bone and cartilage).
In adults, EMT is re-engaged during wound healing, tissue regeneration, organ fibrosis, and cancer progression.
The transforming growth factor-β1 (TGF-β1) cytokine is a strong extracellular inducer of EMT and it was shown to induce EMT phenotypes in prostatic epithelial cells. In follow-up studies, researchers found that TGF-β1 expression was increased with LPS treatment. LPS, short for lipopolysaccharide, is the major component of the outer membrane of Gram-negative bacteria. Researchers also observed that injection of LPS into rat prostates leads to an EMT phenotype.
In this study, a team of researchers hypothesized that an interaction might occur between two signaling pathways: the LPS/TLR4 and TGF-β. Researchers found that LPS/TLR4 signaling exerts a regulatory function on the pseudoreceptor BAMBI (bone morphogenic protein and activin membrane-bound inhibitor), specifically down-regulating its expression. BAMBI is a member of the TGF-β type I receptor family and works as an inhibitor of the TGF-β signaling pathway.
Accordingly, researchers observed that LPS/TLR4 down-regulation of BAMBI enhanced TGF-β signaling in the EMT process in prostatic hyperplasia.
Researchers analyzed TLR4 expression in BPH tissues with inflammation, and found it to be significantly higher, when compared to BPH tissues without inflammation. Correlation studies also suggested TLR4 expression (at the level of messenger RNA) associated with age, BMI, serum PSA levels, urgency and nocturia in the inflammatory group.
In conclusion, these results suggest a link between prostatic hyperplasia and inflammation mediated by modulation of TGF-β induced EMT by LPS/TLR4 axis. Therefore, therapeutic targeting of TLR4 signaling may benefit BPH patients.